FDA Grants Eden Spine 510(K) Clearance for Its New Vertebral Body Replacement
Eden Spine launches in the United States its proprietary Vertebral Body Replacement (VBR), the GIZA™.
Altamonte Springs, FL, February 02, 2012 --(PR.com)-- Eden Spine (www.EdenSpine.com) announced today that it has received FDA 510(k) clearance for its new generation corpectomy device, the GIZA™.
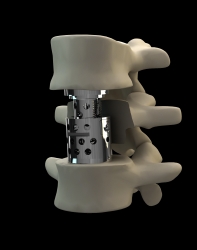
The GIZA™ is an expandable titanium VBR, with rotatable endplates, that provide multiple angulation options by simple endplates rotation. It is intended to replace and fuse a collapsed, damaged, or unstable vertebral body due to a tumor or a fracture.
“Its beauty is its simplicity,” said Mourad Ben Mokhtar, head of Eden Spine’s R&D efforts. He added, "What we did with the GIZA™ is to create an intuitive spinal system designed to help the surgeon easily implant the device, swiftly adapting its height and its angulation to the patient’s characteristics, hence maximizing the chances of a positive clinical outcome.”
For Guillaume Viallaneix, Eden Spine’s CEO, “The approval of the GIZA™ by the FDA is another milestone in the company’s life cycle. The GIZA™ is patented, trademarked, CE Marked, and FDA approved. It enhances the company’s technological footprint and ideally positions Eden Spine for long-term growth. A key 2012 objective is to have the GIZA™ available clinically both in the United States and Internationally via a dedicated network of stocking distributors and strategic partners.”
About Eden Spine:
Eden Spine LLC is a privately held, technology driven, spinal organization. The Eden Spine Group is headquartered in Florida, with a wholly owned subsidiary in Geneva, Switzerland. It is currently developing and distributing a range of new generation spinal technologies. Eden Spine is present in the United States, Europe, the Middle East and Latin America. The company portfolio is currently composed of 5 proprietary technologies; the WELLDISC™ Total Disc Replacement; the PERFX-2™ Dynamic Stabilization System, the WELLEX™ Interspinous Technology, the GIZA™ VBR and the NUMIS™ Lumbar Plating System. Eden Spine currently possesses a range of FDA-cleared and CE Marked spine technologies in the US and internationally.
US Headquarters:
Eden Spine, LLC
377 Maitland Ave - Suite 1015
Altamonte Springs FL 32701
USA
European Offices:
Eden Spine Europe SA
41 rue du 31 Decembre
Geneva 1207
Switzerland
###
The GIZA™ is an expandable titanium VBR, with rotatable endplates, that provide multiple angulation options by simple endplates rotation. It is intended to replace and fuse a collapsed, damaged, or unstable vertebral body due to a tumor or a fracture.
“Its beauty is its simplicity,” said Mourad Ben Mokhtar, head of Eden Spine’s R&D efforts. He added, "What we did with the GIZA™ is to create an intuitive spinal system designed to help the surgeon easily implant the device, swiftly adapting its height and its angulation to the patient’s characteristics, hence maximizing the chances of a positive clinical outcome.”
For Guillaume Viallaneix, Eden Spine’s CEO, “The approval of the GIZA™ by the FDA is another milestone in the company’s life cycle. The GIZA™ is patented, trademarked, CE Marked, and FDA approved. It enhances the company’s technological footprint and ideally positions Eden Spine for long-term growth. A key 2012 objective is to have the GIZA™ available clinically both in the United States and Internationally via a dedicated network of stocking distributors and strategic partners.”
About Eden Spine:
Eden Spine LLC is a privately held, technology driven, spinal organization. The Eden Spine Group is headquartered in Florida, with a wholly owned subsidiary in Geneva, Switzerland. It is currently developing and distributing a range of new generation spinal technologies. Eden Spine is present in the United States, Europe, the Middle East and Latin America. The company portfolio is currently composed of 5 proprietary technologies; the WELLDISC™ Total Disc Replacement; the PERFX-2™ Dynamic Stabilization System, the WELLEX™ Interspinous Technology, the GIZA™ VBR and the NUMIS™ Lumbar Plating System. Eden Spine currently possesses a range of FDA-cleared and CE Marked spine technologies in the US and internationally.
US Headquarters:
Eden Spine, LLC
377 Maitland Ave - Suite 1015
Altamonte Springs FL 32701
USA
European Offices:
Eden Spine Europe SA
41 rue du 31 Decembre
Geneva 1207
Switzerland
###
Contact
Eden Spine, LLC
Guillaume Viallaneix
407-900-9986
www.EdenSpine.com
Guillaume Viallaneix
407-900-9986
www.EdenSpine.com
Categories

