Eden Spine’s Proprietary Dynamic Interspinous Technology and Vertebral Body Replacement Receive Regulatory Approvals in Argentina
“After our successes in Central America, Venezuela and Brazil this is an important step in our continued momentum and commitment in Latin America. We are now actively looking for distributors in Chile, Peru and Columbia."
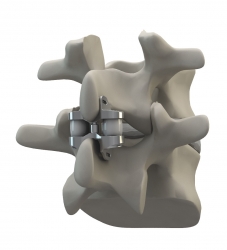
Altamonte Springs, FL, July 31, 2013 --(PR.com)-- Eden Spine is pleased to announce that the WELLEX™ Dynamic Interspinous Technology and the GIZA™ Vertebral Body Replacement have both received regulatory approval in Argentina.
The GIZA™ is an expandable titanium VBR, with rotatable endplates that provide multiple angulation options by simple endplates rotation. It is intended to replace and fuse a collapsed, damaged, or unstable vertebral body due to a tumor or a fracture. The GIZA™ is also approved in the United States, in Europe, and other markets around the world.
The WELLEX™, developed in collaboration with Dr. Jean-Marc Fuentes from France, is a compressible dynamic extension controller that not only relieves pain but also positively affects the long-term health of the segment because of its ability to dynamically control extension while protecting and maintaining the neutral zone. The WELLEX™ has been approved in Europe since 2008, as well as in other international markets.
“We are excited about growing our international distribution network,” says Guillaume Viallaneix, CEO. He adds “After our successes in Central America, Venezuela and Brazil this is an important step in our continued momentum and commitment in Latin America. We are now actively looking for distributors in Chile, Peru and Columbia.”
The GIZA™ is an expandable titanium VBR, with rotatable endplates that provide multiple angulation options by simple endplates rotation. It is intended to replace and fuse a collapsed, damaged, or unstable vertebral body due to a tumor or a fracture. The GIZA™ is also approved in the United States, in Europe, and other markets around the world.
The WELLEX™, developed in collaboration with Dr. Jean-Marc Fuentes from France, is a compressible dynamic extension controller that not only relieves pain but also positively affects the long-term health of the segment because of its ability to dynamically control extension while protecting and maintaining the neutral zone. The WELLEX™ has been approved in Europe since 2008, as well as in other international markets.
“We are excited about growing our international distribution network,” says Guillaume Viallaneix, CEO. He adds “After our successes in Central America, Venezuela and Brazil this is an important step in our continued momentum and commitment in Latin America. We are now actively looking for distributors in Chile, Peru and Columbia.”
Contact
Eden Spine, LLC
Guillaume Viallaneix
407-900-9986
www.EdenSpine.com
Guillaume Viallaneix
407-900-9986
www.EdenSpine.com
Multimedia

CEO Interview
Guillaume Viallaneix shares his vision about the new age of communication in the Medical Devices industry.
Categories

