Perimeter Medical Imaging, Inc. Announces 510(k) Clearance by FDA
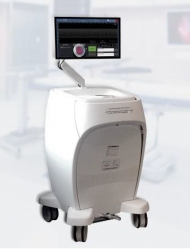
Toronto, Canada, April 09, 2019 --(PR.com)-- Perimeter Medical Imaging, Inc. (“Perimeter Medical”) announced today that the company received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market the second generation of their platform imaging tool used in the evaluation of human tissue microstructure by providing two-dimensional, cross-sectional, real-time depth visualization.
Enhancements to the newly cleared OTIS™ 2.0 system over the previous generation include:
- A novel specimen positioning consumable feature
- Touchscreen interface with improved software, designed to streamline integration with existing clinical workflow
- 10x faster image acquisition speed
Perimeter has developed a world class proprietary reference atlas, with correlations between OTIS™ images and microstructure features identified in histology for a variety of tissue types, including adrenal glands, breast, cervical, colon, heart, kidney, liver, pancreas, spleen, thyroid, tongue and tonsil tissues. This atlas will be available as a tool to clinicians who are learning about the optical coherence tomography imaging modality.
"Today we announce another significant step forward in our mission to provide the best possible tools for clinicians who are treating cancer patients, as well as our preparation for a commercial launch in 2020," said Andrew Berkeley, co-Founder and VP of Business Development of Perimeter Medical. "Our clinical partners want effective, intuitive surgical tools to improve patient care. By assessing subsurface information in real time, clinicians see the potential for better accuracy in intervention. We will continue to advance the field of OCT imaging and expand applications of the OTIS™ system."
The OTIS™ provides high-resolution, real-time imaging of the margin of an excised tissue specimen, enabling clinicians to visualize sub-surface structures up to 2 mm below the surface, while maintaining the integrity of the specimen for standard of care processing, which typically takes place in the hours or days following the surgical procedure.
New World Resource Corp. ("New World" TSX.V “NW”) and Perimeter Medical have previously announced that they entered into a non-binding letter of intent for the acquisition of all of the issued and outstanding shares of Perimeter Medical by New World in a reverse takeover transaction. This transaction will enable Perimeter to raise the capital needed for a full-scale commercial launch in the US in 2020.
Indications for Use: The OTIS™ 2.0 is indicated for use as an imaging tool in the evaluation of excised human tissue microstructure by providing two-dimensional, cross-sectional, real-time depth visualization, with image review manipulation software for identifying and annotating regions of interest.
The OTIS™ 2.0: an advanced imaging tool for excised tissue The first-generation of the OTIS™ platform was the first FDA-cleared device to provide automated, rapid collection of Optical Coherence Tomography (OCT) images from an excised specimen. Conventional OCT imaging devices required the clinician-user to move his/her hand to collect "point-by-point" images, increasing operator workload and generating images from a small number of points on the specimen. The OTIS™ platform leverages Perimeter's proprietary Wide-Field Optical Coherence Tomography Imaging technique to overcome this limitation, leading to rapid, intuitive imaging of the entire specimen.
About Perimeter Medical Imaging, Inc.: Perimeter Medical develops, patents, and commercializes advanced in-procedural medical imaging tools. The OTIS™ platform provides clinicians with real-time, ultra-high resolution, sub-surface image volumes of the margin (1-2 mm below the surface) of an excised tissue specimen. The ability to visualize microscopic tissue structures during the clinical procedure has the potential to result in better long-term outcomes for patients and lower costs to the healthcare system. Perimeter Medical was founded in 2013. Investors, led by Roadmap Capital, have invested over C$16 million in Perimeter Medical that was used for product development, clinical research, and obtaining three FDA clearances. Perimeter has also received C$5 million in non-dilutive funding since 2013.
For information with respect to the Company or the contents of this news release, please email info@perimetermed.com.
Forward Looking Statements: This press release contains forward-looking statements. Statements in this press release that are not purely historical are forward-looking statements. These statements are subject to risks and uncertainties that could cause future results to differ materially from those referenced. Forward-looking statements include, but are not limited to, statements about Perimeter's financial results and market acceptance of Perimeter's existing products, future products or technology. Words such as "could," "anticipates," "expects," "outlook," "intends," "plans," "believes," "seeks," "vision," "estimates," "may," "will," "future," "horizon," "aiming," "driving," "target" (or variations of them) and similar statements, are forward-looking statements. Forward-looking statements involve risks, uncertainties and assumptions that are difficult to predict and could cause Perimeter's results to differ materially from those presented. These risks, uncertainties and assumptions include, but are not limited to, changes in: the regulatory environment; global economics; trade compliance requirements, duties or tariffs; third-party reimbursement levels; currency exchange rates; taxation, healthcare law, and product clearance requirements, as well as those related to: adverse publicity about Perimeter Medical and our products; our reliance on sole or limited source suppliers; our ability to commercialize our products successfully; the impact of competitive products and pricing. These forward-looking statements are made as of the date of this press release, and Perimeter Medical assumes no obligation to update the forward-looking statements, or to update the reasons why actual results differ from those projected in the forward-looking statements, except as required by law
Enhancements to the newly cleared OTIS™ 2.0 system over the previous generation include:
- A novel specimen positioning consumable feature
- Touchscreen interface with improved software, designed to streamline integration with existing clinical workflow
- 10x faster image acquisition speed
Perimeter has developed a world class proprietary reference atlas, with correlations between OTIS™ images and microstructure features identified in histology for a variety of tissue types, including adrenal glands, breast, cervical, colon, heart, kidney, liver, pancreas, spleen, thyroid, tongue and tonsil tissues. This atlas will be available as a tool to clinicians who are learning about the optical coherence tomography imaging modality.
"Today we announce another significant step forward in our mission to provide the best possible tools for clinicians who are treating cancer patients, as well as our preparation for a commercial launch in 2020," said Andrew Berkeley, co-Founder and VP of Business Development of Perimeter Medical. "Our clinical partners want effective, intuitive surgical tools to improve patient care. By assessing subsurface information in real time, clinicians see the potential for better accuracy in intervention. We will continue to advance the field of OCT imaging and expand applications of the OTIS™ system."
The OTIS™ provides high-resolution, real-time imaging of the margin of an excised tissue specimen, enabling clinicians to visualize sub-surface structures up to 2 mm below the surface, while maintaining the integrity of the specimen for standard of care processing, which typically takes place in the hours or days following the surgical procedure.
New World Resource Corp. ("New World" TSX.V “NW”) and Perimeter Medical have previously announced that they entered into a non-binding letter of intent for the acquisition of all of the issued and outstanding shares of Perimeter Medical by New World in a reverse takeover transaction. This transaction will enable Perimeter to raise the capital needed for a full-scale commercial launch in the US in 2020.
Indications for Use: The OTIS™ 2.0 is indicated for use as an imaging tool in the evaluation of excised human tissue microstructure by providing two-dimensional, cross-sectional, real-time depth visualization, with image review manipulation software for identifying and annotating regions of interest.
The OTIS™ 2.0: an advanced imaging tool for excised tissue The first-generation of the OTIS™ platform was the first FDA-cleared device to provide automated, rapid collection of Optical Coherence Tomography (OCT) images from an excised specimen. Conventional OCT imaging devices required the clinician-user to move his/her hand to collect "point-by-point" images, increasing operator workload and generating images from a small number of points on the specimen. The OTIS™ platform leverages Perimeter's proprietary Wide-Field Optical Coherence Tomography Imaging technique to overcome this limitation, leading to rapid, intuitive imaging of the entire specimen.
About Perimeter Medical Imaging, Inc.: Perimeter Medical develops, patents, and commercializes advanced in-procedural medical imaging tools. The OTIS™ platform provides clinicians with real-time, ultra-high resolution, sub-surface image volumes of the margin (1-2 mm below the surface) of an excised tissue specimen. The ability to visualize microscopic tissue structures during the clinical procedure has the potential to result in better long-term outcomes for patients and lower costs to the healthcare system. Perimeter Medical was founded in 2013. Investors, led by Roadmap Capital, have invested over C$16 million in Perimeter Medical that was used for product development, clinical research, and obtaining three FDA clearances. Perimeter has also received C$5 million in non-dilutive funding since 2013.
For information with respect to the Company or the contents of this news release, please email info@perimetermed.com.
Forward Looking Statements: This press release contains forward-looking statements. Statements in this press release that are not purely historical are forward-looking statements. These statements are subject to risks and uncertainties that could cause future results to differ materially from those referenced. Forward-looking statements include, but are not limited to, statements about Perimeter's financial results and market acceptance of Perimeter's existing products, future products or technology. Words such as "could," "anticipates," "expects," "outlook," "intends," "plans," "believes," "seeks," "vision," "estimates," "may," "will," "future," "horizon," "aiming," "driving," "target" (or variations of them) and similar statements, are forward-looking statements. Forward-looking statements involve risks, uncertainties and assumptions that are difficult to predict and could cause Perimeter's results to differ materially from those presented. These risks, uncertainties and assumptions include, but are not limited to, changes in: the regulatory environment; global economics; trade compliance requirements, duties or tariffs; third-party reimbursement levels; currency exchange rates; taxation, healthcare law, and product clearance requirements, as well as those related to: adverse publicity about Perimeter Medical and our products; our reliance on sole or limited source suppliers; our ability to commercialize our products successfully; the impact of competitive products and pricing. These forward-looking statements are made as of the date of this press release, and Perimeter Medical assumes no obligation to update the forward-looking statements, or to update the reasons why actual results differ from those projected in the forward-looking statements, except as required by law
Contact
Perimeter Medical Imaging Inc
Dawna Panas
647-360-0302
www.perimetermed.com
hours of operation - 8-4 Monday - Friday
Dawna Panas
647-360-0302
www.perimetermed.com
hours of operation - 8-4 Monday - Friday
Categories
