BraveHeart Wireless Announces FDA Clearance of the BraveHeart™ Life Sensor Cardiac Monitoring System
The BraveHeart™ Life Sensor Cardiac Monitoring system has been cleared for use in health care settings. The Life Sensor monitoring system securely captures patients’ heart rate and EKG data, and transmits the data to health care providers in real time. More than 28 million Americans diagnosed with heart disease may benefit - with this number growing each year.

Nashua, NH, June 20, 2019 --(PR.com)-- BraveHeart Wireless Inc., a leading innovator in clinical-quality biometric wearables, today announced that the company received clearance from the U.S. Food and Drug Administration (FDA) for the BraveHeart™ Life Sensor Cardiac Monitoring system.
The Life Sensor Cardiac Monitoring system is intended for use by health care professionals within a health care setting. The system captures heart rate and EKG data using a Life Sensor electrode affixed to a patient’s skin and continuously transmits the data from the electrode to an application on an iOS device, where the data can be reviewed by health care professionals.
The Life Sensor Cardiac Monitoring system is a milestone product; it is the first product of its kind built on the BraveHeart Wireless Internet of Things (IoT) “open” platform. BraveHeart Wireless continues to develop additional health-related products on this platform as it works closely with key partners to configure its platform for targeted use models.
“Prior to the Life Sensor, people with the need for truly wireless heart-monitoring had limited options. Now, health care professionals can use the Life Sensor to quickly and wirelessly receive relevant, clinical-quality and actionable heart rate and EKG data - even as a person is waiting to be admitted to a health care facility,” said Steve McCalmont, CEO of BraveHeart Wireless Inc.
“The Life Sensor has the potential to significantly and positively impact patient care,” said Dave Shimkus, Vice President of Operations at BraveHeart Wireless Inc. “The FDA ensured the Life Sensor cardiac monitoring system meets the FDA’s regulatory requirements for safety and effectiveness. We look forward to seeing the BraveHeart Life Sensor platform widely used in a variety of health care settings, and to working with our partners to develop additional products.”
About The BraveHeart Life Sensor Cardiac Monitoring System
The Life Sensor Cardiac Monitoring system introduces a proprietary and pioneering set of features to the cardiac monitoring segment, including these key features:
- Everyday-wear form factor. The Life Sensor Cardiac Monitoring system is the smallest, most comfortable, clinical-quality sensor patch on the market.
- Patent-pending technology. The Life Sensor Cardiac Monitoring system employs both passive and active sensors. Initial patents have been filed on BraveHeart Wireless’s critical method of multiplexing multiple sensors on a single patch.
About The BraveHeart Life Sensor IoT Open Platform
The BraveHeart Life Sensor Internet of Things open platform is used to power the FDA-cleared Life Sensor Cardiac Monitoring system. The platform’s additional features include:
- OEM / partner channel model. The seamless integration potential of the Life Sensor Cardiac Monitoring system is designed to accelerate its penetration into multiple medical segments, from the solo researcher to large medical device companies.
- Widest array of sensors on a single patch. Because of the number and type of sensors that are built into the Life Sensor system, health care partners can rely on the Life Sensor to cover patient needs in multiple health care sectors.
- Open-platform. The Life Sensor Cardiac Monitoring system harnesses a modern, cloud-based RESTful API and Modular hardware platform. The Life Sensor Cardiac Monitoring system is designed to transmit continuous data to enable telemedicine and other modalities.
Life Sensor Cardiac Monitoring System Indications
In the United States, 28.2 million adults are currently diagnosed with heart disease - and this number continues to grow. Every year, about 735,000 Americans suffer a heart attack, and heart disease has now become the leading cause of death for men and women in the United States, according to the Centers for Disease Control.
The chances of survival are greater when emergency treatment begins quickly. The Life Sensor Cardiac Monitoring system is designed to provide fast application and access to a patient’s heart rate and EKG data.
The Life Sensor Cardiac Monitoring system is comprised of several components, including a single-use, self-adhesive patch that can be attached to a patient’s skin directly above the heart. This patch is equipped with a sensor that can capture a patient’s heart rate and EKG data and wirelessly transmit this data via BLE (Bluetooth Low Energy) in real-time to an application on an iOS device, where the data can be reviewed by health care professionals.
About BraveHeart Wireless
BraveHeart Wireless Inc. is a privately held medical device company focused on developing and commercializing innovative technologies. The company is headquartered in Nashua, New Hampshire. For further information, visit www.BraveHeart.life. Follow us on Twitter at @BraveHeartTech and on Facebook at @BraveHeartWireless.
BraveHeart™ Life Sensor is a trademark of BraveHeart Wireless Inc.
About The FDA
The FDA, an agency within the U.S. Department of Health and Human Services, promotes and protects the public health by, among other things, assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
The Life Sensor Cardiac Monitoring system is intended for use by health care professionals within a health care setting. The system captures heart rate and EKG data using a Life Sensor electrode affixed to a patient’s skin and continuously transmits the data from the electrode to an application on an iOS device, where the data can be reviewed by health care professionals.
The Life Sensor Cardiac Monitoring system is a milestone product; it is the first product of its kind built on the BraveHeart Wireless Internet of Things (IoT) “open” platform. BraveHeart Wireless continues to develop additional health-related products on this platform as it works closely with key partners to configure its platform for targeted use models.
“Prior to the Life Sensor, people with the need for truly wireless heart-monitoring had limited options. Now, health care professionals can use the Life Sensor to quickly and wirelessly receive relevant, clinical-quality and actionable heart rate and EKG data - even as a person is waiting to be admitted to a health care facility,” said Steve McCalmont, CEO of BraveHeart Wireless Inc.
“The Life Sensor has the potential to significantly and positively impact patient care,” said Dave Shimkus, Vice President of Operations at BraveHeart Wireless Inc. “The FDA ensured the Life Sensor cardiac monitoring system meets the FDA’s regulatory requirements for safety and effectiveness. We look forward to seeing the BraveHeart Life Sensor platform widely used in a variety of health care settings, and to working with our partners to develop additional products.”
About The BraveHeart Life Sensor Cardiac Monitoring System
The Life Sensor Cardiac Monitoring system introduces a proprietary and pioneering set of features to the cardiac monitoring segment, including these key features:
- Everyday-wear form factor. The Life Sensor Cardiac Monitoring system is the smallest, most comfortable, clinical-quality sensor patch on the market.
- Patent-pending technology. The Life Sensor Cardiac Monitoring system employs both passive and active sensors. Initial patents have been filed on BraveHeart Wireless’s critical method of multiplexing multiple sensors on a single patch.
About The BraveHeart Life Sensor IoT Open Platform
The BraveHeart Life Sensor Internet of Things open platform is used to power the FDA-cleared Life Sensor Cardiac Monitoring system. The platform’s additional features include:
- OEM / partner channel model. The seamless integration potential of the Life Sensor Cardiac Monitoring system is designed to accelerate its penetration into multiple medical segments, from the solo researcher to large medical device companies.
- Widest array of sensors on a single patch. Because of the number and type of sensors that are built into the Life Sensor system, health care partners can rely on the Life Sensor to cover patient needs in multiple health care sectors.
- Open-platform. The Life Sensor Cardiac Monitoring system harnesses a modern, cloud-based RESTful API and Modular hardware platform. The Life Sensor Cardiac Monitoring system is designed to transmit continuous data to enable telemedicine and other modalities.
Life Sensor Cardiac Monitoring System Indications
In the United States, 28.2 million adults are currently diagnosed with heart disease - and this number continues to grow. Every year, about 735,000 Americans suffer a heart attack, and heart disease has now become the leading cause of death for men and women in the United States, according to the Centers for Disease Control.
The chances of survival are greater when emergency treatment begins quickly. The Life Sensor Cardiac Monitoring system is designed to provide fast application and access to a patient’s heart rate and EKG data.
The Life Sensor Cardiac Monitoring system is comprised of several components, including a single-use, self-adhesive patch that can be attached to a patient’s skin directly above the heart. This patch is equipped with a sensor that can capture a patient’s heart rate and EKG data and wirelessly transmit this data via BLE (Bluetooth Low Energy) in real-time to an application on an iOS device, where the data can be reviewed by health care professionals.
About BraveHeart Wireless
BraveHeart Wireless Inc. is a privately held medical device company focused on developing and commercializing innovative technologies. The company is headquartered in Nashua, New Hampshire. For further information, visit www.BraveHeart.life. Follow us on Twitter at @BraveHeartTech and on Facebook at @BraveHeartWireless.
BraveHeart™ Life Sensor is a trademark of BraveHeart Wireless Inc.
About The FDA
The FDA, an agency within the U.S. Department of Health and Human Services, promotes and protects the public health by, among other things, assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Contact
BraveHeart Wireless Inc.
Laurie Dove
316-305-5245
braveheart.life
Laurie Dove
316-305-5245
braveheart.life
Multimedia
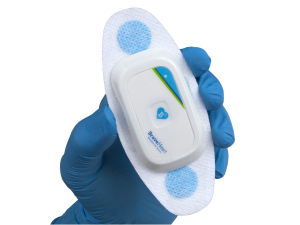
BraveHeart(TM) Life Sensor Cardiac Monitoring System
The BraveHeart(TM) Life Sensor Cardiac Monitoring system has been cleared for use in health care settings.
Categories
