New Treatment Shows Vision Benefit in Age Related Macular Degeneration
MD Stem Cells reports results for Dry AMD from their clinical study SCOTS - the Stem Cell Ophthalmology Treatment Study. 63% of treated and evaluated eyes achieved improvement in vision while an additional 34% had vision remain stable in follow up. No complications occurred. Results were statistically significant.
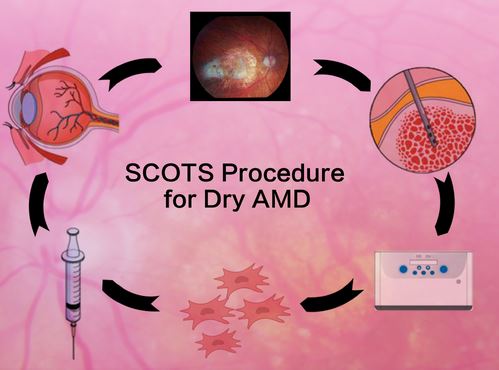
Westport, CT, April 18, 2020 --(PR.com)-- Age Related Macular Degeneration - specifically dry AMD - will affect almost 200 million people worldwide in 2020. An approach, pioneered by MD Stem Cells, using bone marrow stem cells from the actual patient has now shown visual improvement for 63% of dry AMD eyes and stability in another 34%, demonstrating statistical significance in helping patients with this blinding disease. Results were recently published in the Medicines Journal - a highly regarded international medical journal. The title of the paper: Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Age-Related Macular Degeneration.
There is no FDA approved medication for treating dry AMD. Certain vitamins may reduce the risk of bleeding or wet AMD. But vitamins do not appear to stop the relentless loss of retinal cells and vision called geographic atrophy (GA) from AMD.
The highlight of the MD Stem Cell report was that 63% of dry AMD eyes treated and followed had vision improvement. This ranged from 2.5% to 44.6% with an average of 27.6% on a scientific vision scale called LogMAR. An additional 34% of eyes remained stable for the follow up period- important because many of the eyes had previously been losing vision. The findings were highly statistically significant with p < 0.001 meaning that the results overwhelmingly met that medical standard and confirming that the BMSC treatment was responsible for the improvements seen.
The Stem Cell Treatment Ophthalmology Study- both SCOTS and SCOTS2 - has been treating many different eye diseases since 2013 using the patient’s own bone marrow stem cells (BMSC) injected in the orbit around the eye. The study is Institutional Board Approved and National Institutes of Health registered on www.clinicaltrials.gov NCT 03011541. The physicians involved with MD Stem Cells now have 14 world class medical and scientific publications primarily reporting their clinical results in ophthalmology. This is vastly more than any other stem cell research group working with eye disease and should be reassuring to patients and health care providers seeking treatment options. Different optic nerve diseases, NAION, LHON, DOA, optic atrophy; as well as several retinal diseases including Retinitis Pigmentosa, Ushers and now AMD have all shown benefit. MD Stem Cells has worked to achieve the safest, most effective approach to dry AMD using BMSC - with gratifying success.
“Following multiple patient treatments and over a dozen peer-reviewed papers, our studies have shown that a patient’s own bone marrow stem cells (BMSC) can have positive effects on different retinal and optic nerve diseases,” explains Dr. Levy, CEO and Chief Science Officer for MD Stem Cells. “As we have continued the study, other researchers have published numerous papers revealing how this may be occurring: release of exosomes with neurotrophic factors helping neurons and photoreceptors, transfer of cytoplasmic structures such as mitochondria to injured cells, and transdifferentiation of BMSC into neurons.”
Dr. Levy concludes: “The research has shown that patients with dry AMD choosing to participate in the SCOTS 2 may have a significant likelihood of either improving or stabilizing their vision.”
Patients may receive information about SCOTS 2 by emailing stevenlevy@mdstemcells.com, using the contact us page on www.mdstemcellscom, or calling 203-423-9494. The Stem Cell Ophthalmology Study 2 is enrolling patients with different retina and optic nerve diseases. MD Stem Cells has no grant support and is not a pharmaceutical company; these are patient sponsored studies and the patients pay for both treatment and travel.
There is no FDA approved medication for treating dry AMD. Certain vitamins may reduce the risk of bleeding or wet AMD. But vitamins do not appear to stop the relentless loss of retinal cells and vision called geographic atrophy (GA) from AMD.
The highlight of the MD Stem Cell report was that 63% of dry AMD eyes treated and followed had vision improvement. This ranged from 2.5% to 44.6% with an average of 27.6% on a scientific vision scale called LogMAR. An additional 34% of eyes remained stable for the follow up period- important because many of the eyes had previously been losing vision. The findings were highly statistically significant with p < 0.001 meaning that the results overwhelmingly met that medical standard and confirming that the BMSC treatment was responsible for the improvements seen.
The Stem Cell Treatment Ophthalmology Study- both SCOTS and SCOTS2 - has been treating many different eye diseases since 2013 using the patient’s own bone marrow stem cells (BMSC) injected in the orbit around the eye. The study is Institutional Board Approved and National Institutes of Health registered on www.clinicaltrials.gov NCT 03011541. The physicians involved with MD Stem Cells now have 14 world class medical and scientific publications primarily reporting their clinical results in ophthalmology. This is vastly more than any other stem cell research group working with eye disease and should be reassuring to patients and health care providers seeking treatment options. Different optic nerve diseases, NAION, LHON, DOA, optic atrophy; as well as several retinal diseases including Retinitis Pigmentosa, Ushers and now AMD have all shown benefit. MD Stem Cells has worked to achieve the safest, most effective approach to dry AMD using BMSC - with gratifying success.
“Following multiple patient treatments and over a dozen peer-reviewed papers, our studies have shown that a patient’s own bone marrow stem cells (BMSC) can have positive effects on different retinal and optic nerve diseases,” explains Dr. Levy, CEO and Chief Science Officer for MD Stem Cells. “As we have continued the study, other researchers have published numerous papers revealing how this may be occurring: release of exosomes with neurotrophic factors helping neurons and photoreceptors, transfer of cytoplasmic structures such as mitochondria to injured cells, and transdifferentiation of BMSC into neurons.”
Dr. Levy concludes: “The research has shown that patients with dry AMD choosing to participate in the SCOTS 2 may have a significant likelihood of either improving or stabilizing their vision.”
Patients may receive information about SCOTS 2 by emailing stevenlevy@mdstemcells.com, using the contact us page on www.mdstemcellscom, or calling 203-423-9494. The Stem Cell Ophthalmology Study 2 is enrolling patients with different retina and optic nerve diseases. MD Stem Cells has no grant support and is not a pharmaceutical company; these are patient sponsored studies and the patients pay for both treatment and travel.
Contact
MD Stem Cells
Steven Levy MD
203-423-9494
www.mdstemcells.com
Steven Levy MD
203-423-9494
www.mdstemcells.com
Categories
