MedicaMetrix Announces CE Mark Approval for ProstaMetrix
This next-generation technology allows physicians to accurately measure the volume of the prostate gland using a minimally invasive medical device.
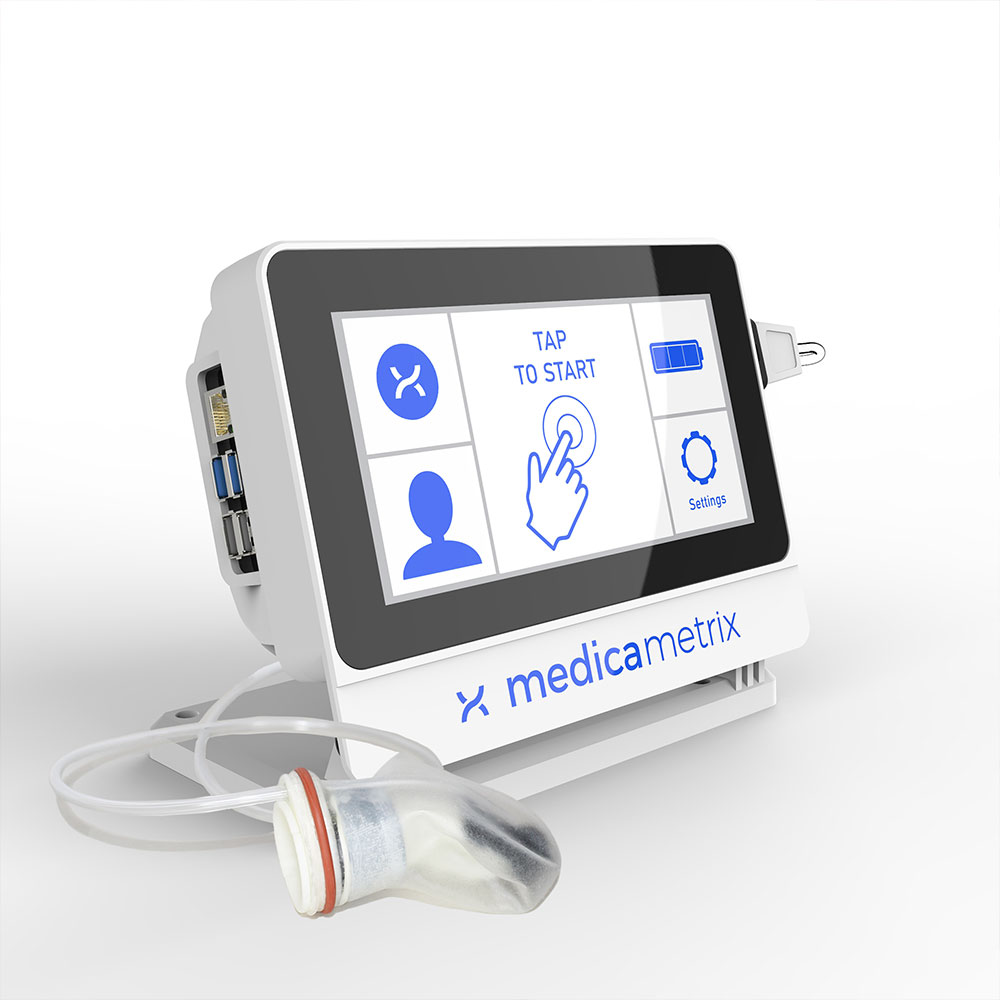
Boston, MA, September 08, 2021 --(PR.com)-- MedicaMetrix, Inc., today announced receiving CE mark approval for ProstaMetrix™, a minimally invasive medical device utilizing a fingertip optical encoder, associated controller and battery pack. This approval allows MedicaMetrix to progress its plans for international expansion.
ProstaMetrix enables early evaluation of prostate health through accurate volumetric measurements, differentiating patients at higher risk and mitigating uncertainty. Patients benefit by avoiding invasive, higher-risk procedures, having better care management for both prostate cancer and BPH and thus, a better quality of life.
The CE marking confirms the ProstaMetrix system meets all the requirements of the European Medical Devices Directive and follows the company’s recent ISO 13485:2016 certification for Medical Device and Quality Management Systems.
“CE mark approval allows MedicaMetrix to market and distribute ProstaMetrix in the European Union and many other countries around the world that recognize the CE mark,” Satish Vankayalapati, Chairman and Founder of MedicaMetrix stated. “We have reached another significant milestone as we work to advance prostate health management globally.”
ProstaMetrix’s multiple technological innovations allow it to:
- Identify less invasive diagnostic and treatment options for patients, including active surveillance.
- Identify higher-risk patients for earlier intervention and biopsy as needed.
- Ability to focus biopsies and invasive therapies on patients that need it.
“The ProstaMetrix device will truly redefine how patients and physicians view men’s health generally and the treatment of prostate cancer specifically,” said Peter Madras, M.D., Chief Medical Officer of MedicaMetrix. “Because our device transforms the physical examination of the prostate into a quantitative, reproducible measurement in a manner completely transparent to the patient, with instant information, doctors and their patients will be better informed and empowered to make the best treatment plans.”
About MedicaMetrix
MedicaMetrix develops innovative technologies and device solutions that transform the healthcare status quo, leading to better medical outcomes, streamlined care and enhanced patient experience.
MedicaMetrix is leading the development of a new paradigm that transforms the diagnosis, treatment, and management of prostate health by filling the gap between PSA testing and imaging / biopsies.
MedicaMetrix is planning to acquire and develop other new medical devices with the goal to bring them to market rapidly by leveraging planned production facilities in the U.S. and India.
Cautionary Statements Concerning Forward-Looking Statements
Some of the statements in this press release may be forward-looking statements or statements of future expectations based on currently available information. Such statements are naturally subject to risks and uncertainties. Factors such as the development of general economic conditions, future market conditions, unusual catastrophic loss events, changes in the capital markets and other circumstances may cause the actual events or results to be materially different from those anticipated by such statements. MedicaMetrix Inc. does not make any representation or warranty, express or implied, as to the accuracy, completeness, or updated status of such statements. Therefore, in no case whatsoever will MedicaMetrix Inc. and its affiliate companies be liable to anyone for any decision made or action taken in conjunction with the information and/or statements in this press release or for any related damages.
MedicaMetrix, Inc.
ProstaMetrix enables early evaluation of prostate health through accurate volumetric measurements, differentiating patients at higher risk and mitigating uncertainty. Patients benefit by avoiding invasive, higher-risk procedures, having better care management for both prostate cancer and BPH and thus, a better quality of life.
The CE marking confirms the ProstaMetrix system meets all the requirements of the European Medical Devices Directive and follows the company’s recent ISO 13485:2016 certification for Medical Device and Quality Management Systems.
“CE mark approval allows MedicaMetrix to market and distribute ProstaMetrix in the European Union and many other countries around the world that recognize the CE mark,” Satish Vankayalapati, Chairman and Founder of MedicaMetrix stated. “We have reached another significant milestone as we work to advance prostate health management globally.”
ProstaMetrix’s multiple technological innovations allow it to:
- Identify less invasive diagnostic and treatment options for patients, including active surveillance.
- Identify higher-risk patients for earlier intervention and biopsy as needed.
- Ability to focus biopsies and invasive therapies on patients that need it.
“The ProstaMetrix device will truly redefine how patients and physicians view men’s health generally and the treatment of prostate cancer specifically,” said Peter Madras, M.D., Chief Medical Officer of MedicaMetrix. “Because our device transforms the physical examination of the prostate into a quantitative, reproducible measurement in a manner completely transparent to the patient, with instant information, doctors and their patients will be better informed and empowered to make the best treatment plans.”
About MedicaMetrix
MedicaMetrix develops innovative technologies and device solutions that transform the healthcare status quo, leading to better medical outcomes, streamlined care and enhanced patient experience.
MedicaMetrix is leading the development of a new paradigm that transforms the diagnosis, treatment, and management of prostate health by filling the gap between PSA testing and imaging / biopsies.
MedicaMetrix is planning to acquire and develop other new medical devices with the goal to bring them to market rapidly by leveraging planned production facilities in the U.S. and India.
Cautionary Statements Concerning Forward-Looking Statements
Some of the statements in this press release may be forward-looking statements or statements of future expectations based on currently available information. Such statements are naturally subject to risks and uncertainties. Factors such as the development of general economic conditions, future market conditions, unusual catastrophic loss events, changes in the capital markets and other circumstances may cause the actual events or results to be materially different from those anticipated by such statements. MedicaMetrix Inc. does not make any representation or warranty, express or implied, as to the accuracy, completeness, or updated status of such statements. Therefore, in no case whatsoever will MedicaMetrix Inc. and its affiliate companies be liable to anyone for any decision made or action taken in conjunction with the information and/or statements in this press release or for any related damages.
MedicaMetrix, Inc.
Contact
Medica Metrix Inc.
Jamie Jamitkowski
978-557-0794
www.medicametrix.com
Jamie Jamitkowski
978-557-0794
www.medicametrix.com
Categories
