Recent Headlines
Within Biotechnology
NuvOx Pharma LLC Announces Authorization of ARDS Trial from Health Canada
NuvOx Pharma LLC (“NuvOx”) announced that it has received authorization from Health Canada for its EXTEND: novEl oXygen ThErapeutic NanO2 for mild respiratory distress in Phase Ib and ARDS in Phase II Trials. Evan Unger, MD, CEO of NuvOx, said, “Acute Respiratory Distress... - July 12, 2024 - NuvOx Therapeutics
Abel Womack CEO Announces His Retirement and Successor
After 50 years with Abel Womack, John Croce, CEO retires. - June 27, 2024 - Abel Womack Inc
Daicel Arbor Biosciences Launches New myTXTL® Kits to Accelerate Protein Expression
Daicel Arbor Biosciences is excited to announce the release of the next generation of its myTXTL® kits, for cell-free protein expression, designed to simplify and accelerate antibody discovery and protein engineering. These powerful new kits include the myTXTL Pro Kit and the myTXTL Antibody/DS... - June 27, 2024 - Daicel Arbor Biosciences
SYNAPS Dx Announces Expansion to New, State-of-the-Art Facility
SYNAPS Dx has moved to a new, state-of-the-art facility in Rockville, MD, to meet growing demand and enhance patient care. The larger space and advanced infrastructure will support more diagnostic samples and ensure timely, accurate Alzheimer's and dementia diagnoses. Fully qualified for high-complexity clinical lab testing, the facility boosts diagnostic capabilities. The expansion will also advance the DISCERN™ test, a minimally invasive tool distinguishing Alzheimer's from other dementias. - June 26, 2024 - SynapsDx
Cambridge Polymer Group Unveils Restructure to Accelerate Client Innovation through Material Science
Cambridge Polymer Group (CPG) announced a new leadership structure to better support clients in developing innovative products. CPG is creating a new five-person senior management team with deep materials science expertise, which will allow for closer collaboration with clients on complex projects. Dr. Gavin Braithwaite will be the new CEO and Dr. Stephen Spiegelberg will move to the new role of Chief Scientific Officer (CSO). - June 23, 2024 - Cambridge Polymer Group
KIRON.AI Launches Book “NEOBANK Navigating the Future of Banking” by Author and Bank Owner Ronald Ingram
KIRON.AI, a Henderson, Nevada-based AI Health & Wealth company launches “NEOBANK Navigating the Future of Banking,” a groundbreaking book exposing the history and predicting the future of banking from author Ronald Ingram’s perspective as a bank owner, historian, futurist and polymath. The company KIRON.AI employs artificial intelligence (AI) to discover, uncover, develop and deploy protocols, tools and information to enhance human wealth and health preservation and generation. - June 11, 2024 - Kiron.AI
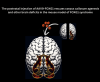
Groundbreaking Study Shows Promise in AAV9 Gene Therapy for FOXG1 Syndrome; Rescue of Brain Structure Abnormalities and Deficits.
New publication shows groundbreaking result in FOXG1 AAV9 gene therapy studies, rescuing structural brain abnormalities caused by the pediatric rare disease, FOXG1 syndrome. This paper summarizing work done by the FOXG1 Research Center, led by Dr. Soo-Kyung Lee, Dr. Jae W. Lee and Dr. Kathrin Meyer (responsible for the SMA gene therapy), shows remarkable rescue of brain abnormalities using AAV9 gene therapy. - June 10, 2024 - FOXG1 Research Foundation
Cayman Chemical Expands Molecular Diagnostic Research Offerings Through Partnership with PCRassays.com
Cayman Chemical, a leading supplier of research tools for the life science community, has widened their portfolio to include molecular-based detection kits through a strategic partnership with PCRassays.com. PCRassays.com offers more than 200 qPCR-based assays for infectious diseases, over 50 of... - June 07, 2024 - Cayman Chemical Company
FlowSense: Affordable Antibiotic Testing to Transform the Honey Industry from Biorex Food Diagnostics
Biorex Food Diagnostics announced today the launch of its cutting-edge FlowSense product line, with the first phase designed specifically for the honey industry. The new FlowSense test products introduce a comprehensive range of antibiotic test panels, providing honey producers with an innovative... - June 07, 2024 - Biorex Food Diagnostics
Limited Opportunity: Enroll in Ales for ALS 2024 Today, Secure Your Spot as Part of a Growing National Movement
Ales for ALS invites brewers to craft distinctive and flavorful beers using a custom hop blend generously donated by Yakima Chief Hops. In exchange, participating brewers pledge to donate $1 for every pint sold, directly contributing to groundbreaking ALS research at the ALS Therapy Development Institute (ALS TDI). - May 04, 2024 - ALS Therapy Development Institute
Revolutionizing Clinical Trials: AnzuBridge Unveils Next-Generation Solutions for Improved Efficacy Evaluations in the Treatment of Rare Disease
AnzuBridge® updates its CDMS to enhance biotech evaluations with advanced video tools, ensuring precise efficacy recording and compliance. It supports scalable trials and features HIPAA-compliant media capture, ePRO for patient engagement, and sophisticated video review capabilities, all integrated with a Learning Management System for efficient trial management. - April 30, 2024 - Anzu
No More Acetone: Thar Process Develops Clean and Green Process to Create Sterile, Fat-Free Lecithin Powder and GMP Quality Oils for Human Consumption
Thar Process, with 34 years of expertise in supercritical fluid extraction, has developed a sustainable way to produce de-fatted lecithin powder that has less oil and longer shelf-life stability. The new process is semi-continuous and sustainable because it utilizes upcycled CO2 from waste sources instead of using toxic acetone which is the industry standard. - April 26, 2024 - Thar Process
Revvity’s EUROIMMUN and ALPCO-GeneProof Announce Strategic Partnership to Expand CE-IVD Molecular Assay Offerings
Revvity’s EUROIMMUN business, a leading provider of high-quality in-vitro diagnostic products, and ALPCO-GeneProof, a global leader in molecular diagnostics, jointly announced a strategic partnership to enhance the availability of GeneProof PCR kits throughout the European Union. This... - April 25, 2024 - ALPCO
Mirror Biologics, Inc. Appoints Elizabeth Czerepak as Chief Financial Officer and VP Corporate Development
Mirror Biologics, Inc., a clinical stage biopharmaceutical company announces the appointment of Elizabeth Adkins Czerepak as Chief Financial Officer (CFO) and Vice President of Corporate Development effective April 15, 2024. Ms. Czerepak has served as CFO of several biotechnology companies over... - April 18, 2024 - Mirror Biologics, Inc.
Huntington Study Group Launches Project AWARE 2.0 Observational Study
The Huntington Study Group® and HSG Clinical Research, Inc. (together, “HSG”) are pleased to announce the launch of Project AWARE 2.0, an observational study developed in collaboration with Roche Products Limited and Genentech to understand and improve Awareness, Willingness, and... - April 10, 2024 - Huntington Study Group
Minus K Congratulates to the Winners of Their 2023/2024 Educational Giveaway
Minus K congratulates the four winners of the Minus K's 2023/2024 Educational Giveaway. Over $75,000 of new passive mechanical hub hertz vibration isolators have been granted to universities and colleges in the U.S. over the last ten years. - April 10, 2024 - Minus K Technology Inc.
FuseBio Presents New Data at AACR: Targeting a Functionally-Selective IL-18 (F-18) to PD-1: A Next Generation Checkpoint Inhibitor with Enhanced Anti-Tumor Activity
FuseBio's unique attenuation mechanism renders IL-18, a functionally selective IL-18, resistant to IL-18BP and 1 million percent less active in circulation and yet still retain nearly full activity when targeted to PD-1+/IL-18R+ cells, the key functional immune cells in tumors. It is designed to retain full activity of PD-1 checkpoint blockade and simultaneously deliver a functionally selective IL-18 (F-18) to preserve and enhance the anti-tumor activity of immune cells. - April 09, 2024 - Fuse Biotherapeutics
Ngram Expands AI Dataset Offerings with Descriptive Question Answering for Healthcare
New open-source medchat-qa-descriptive dataset enables AI models to provide comprehensive answers to complex medical queries. - March 22, 2024 - ngram Inc.
Parvus Announces Collaboration with AbbVie to Develop IBD Therapies Based on the Parvus Navacim Platform Technology
The collaboration will combine AbbVie’s expertise in Immunology with Parvus’ innovative Navacim™ regulatory T cells (Treg) immune tolerization platform technology to develop therapies for Inflammatory Bowel Disease Parvus Therapeutics announced today that it has entered into an... - March 20, 2024 - Parvus Therapeutics U.S., Inc.
MENADNA and ProPhase Labs' (NASDAQ: PRPH) Wholly-Owned Subsidiary, Nebula Genomics, Announce Strategic Partnership to Enhance Genomic Testing in Jordan, Oman and Iraq
MENADNA Inc., has entered into an exclusive partnership with ProPhase Labs' subsidiary, Nebula Genomics, aiming to advance genomic testing in Jordan, Oman, and Iraq. This collaboration will leverage Nebula Genomics' advanced sequencing technologies and MENADNA's bioinformatics capabilities to improve the quality and relevance of genomic data in these historically underserved regions. - March 18, 2024 - MENADNA
iView Therapeutics Inc. Announced FDA’s Clearance of IND Application for IVW-1001 Ophthalmic Eyelid Wipe to Treat Signs and Symptoms of Dry Eye Disease
iView Therapeutics Inc., a clinical stage biotechnology company dedicated to advancing innovative treatments for ocular diseases, announces that the U.S. Food and Drug Administration (FDA) cleared iView’s Investigational New Drug (IND) application to for the initiation of a Phase 1/2 clinical... - March 11, 2024 - iView Therapeutics Inc.
Defined Bioscience Introduces Ready-CEPT™: A New Cell Viability Enhancer
Defined Bioscience is pleased to announce the launch of Ready-CEPT™ 1000X, a new product designed to improve stem cell survival, growth, and health during critical processes such as routine passaging, cryopreservation, and freeze-thaw recovery. - March 11, 2024 - Defined Bioscience Inc.
ALS TDI Launches ALS Trial Navigator: A Revolutionary Platform to Simplify Access to Clinical Trials
The ALS Therapy Development Insitute (ALS TDI) has announced the beta launch of the ALS Trial Navigator. This innovative set of tools is designed to guide people with ALS and caregivers through the process of finding and understanding ALS clinical trials – with the ultimate goal of personalizing the search process and helping users make informed decisions about participation in trials. - February 29, 2024 - ALS Therapy Development Institute
IVIEW Announces Presentation of Positive Topline Results in Phase II Clinical Study of IVIEW-1201 for the Treatment of Bacterial Conjunctivitis at ARVO 2024
IVIEW Therapeutics Inc. announces to present the positive topline result in the Phase II Clinical Study of IVIEW-1201 for the Treatment of Bacterial Conjunctivitis in the upcoming 2024 ARVO (The Association for Research in Vision and Ophthalmology) national meeting in Seattle, WA. The Presentation... - February 28, 2024 - iView Therapeutics Inc.
Cayman Chemical Introduces LipidLaunch™, Expands Portfolio of Lipid Nanoparticle Research Tools to Support Advances in RNA Therapies
Cayman Chemical introduces the latest addition to the company’s portfolio of lipid nanoparticle (LNP) research tools for academic, pharma, and biotech researchers worldwide. The LipidLaunch™ product line helps researchers pursue LNPs for the delivery of nucleic acid therapies in untold health applications, ranging from vaccine development to cancer and cell or gene therapies. - February 22, 2024 - Cayman Chemical Company
Biopharmaceutical Sponsor Interaction with Clinical Site Networks Pulse Report
Sponsor Outsourcing Dollars May Shift to Clinical Site Networks, Transforming the CRO Relationship - February 13, 2024 - Life Science Strategy Group, LLC
Microvascular Therapeutics Welcomes Thom Tulip as Chief Business Officer
Microvascular Therapeutics Inc. (MVT), a clinical-stage biotech company at the forefront of developing the next generation of microbubbles unlocking the therapeutic potential of ultrasound, proudly announces the appointment of Dr Thom Tulip as their new Chief Business Officer. In this pivotal role, Dr Tulip will lead capital raises to propel MVT's cutting-edge technologies into the market. - February 10, 2024 - Microvascular Therapeutics, Inc.
Flow Circuits and Rapid Fluidics Ltd. Announce Partnership to Drive Microfluidic Innovation
Flow Circuits design platform and Rapid Fluidics 3D Print capabilities perfectly complement each other for rapid fluidic system development. Combined they empower researchers in diverse markets such as Pharmaceutical & Life Sciences Research, Point of Care Testing and Clinical Diagnostics, and Environment and Industrial Analytics. - February 05, 2024 - Rapid Fluidics Ltd
Creative Diagnostics Announces New AAV Reagent Solutions for Gene Therapy
Creative Diagnostics has announced its extensive portfolio of AAV Reagent Solutions to support researchers. - February 04, 2024 - Creative Diagnostics
Creative Diagnostics Launches Serum Plate Agglutination Test for In Vitro Detection of Multiple Pathogens
Creative Diagnostics is pleased to announce the launch of its Serum Plate Agglutination Test. - February 04, 2024 - Creative Diagnostics
Creative Diagnostics Launches Aflatoxin Test Reagents to Ensure Food Safety
Creative Diagnostics has announced the launch of Aflatoxin Test Reagents to detect harmful mycotoxins in various food products. - February 04, 2024 - Creative Diagnostics
Patient Experience and Satisfaction with Electronic Consent, Onboarding, and Diaries in Clinical Trials Report
New Market Research Shows Different Patient Preferences for Electronic Diaries - January 31, 2024 - Life Science Strategy Group, LLC
New Sustainable Lyophilization Technology Uses Supercritical CO2
The Thar group of companies have been using CO2 for extraction, purification, particle design and encapsulation for many years but recent developments point to a need for sustainable methods for drying APIs, intermediates, excipients and biologics at relatively low temperatures. 'Big Pharma' has depended on Thar for SFC tools in drug discovery but lyophilization will likely be used in manufacturing too. - January 28, 2024 - Thar Process
Creative Diagnostics Announces MDT Services for Measuring Antibacterial Effectiveness
Creative Diagnostics has announced new Minimum Doubling Time (MDT) services to measure the effectiveness of antibacterial compounds. - January 26, 2024 - Creative Diagnostics
Creative Diagnostics Launches New P53 and Tp53 Antibodies for Cancer Research
Creative Diagnostics has announced P53 and TP53 antibodies and solutions to support cancer research. - January 26, 2024 - Creative Diagnostics
Creative Diagnostics Launches Rapid Robenidine Test Reagents for the Analysis of Anticoccidial Residues
Creative Diagnostics has announced the launch of its new Robenidine Test Reagents for the detection of robenidine residues. - January 26, 2024 - Creative Diagnostics
Huntington Study Group Announces 2024 Annual Meeting
The Huntington Study Group® (HSG), together with its wholly owned subsidiary, HSG Clinical Research, Inc., a world leader in conducting clinical trials for Huntington’s disease (HD), is pleased to announce that its annual meeting for 2024 is slated to take place from November 6-9 at the... - January 22, 2024 - Huntington Study Group
Impact of the In Vitro Diagnostic Regulation on Clinical Trials in the EU and CRO Outsourcing Report
IVDR Delays Driving Trial Sponsors to Actively Seek Alternative Site and CRO Outsourcing Strategies - January 17, 2024 - Life Science Strategy Group, LLC
NuvOx Pharma Announces Issuance of a New Patent
NuvOx Pharma has been issued US Patent No. 11,857,627, entitled “Fractionated Radiotherapy and Chemotherapy with an Oxygen Therapeutic.” - January 14, 2024 - NuvOx Therapeutics
New Ultra-Thin, Compact Low-Height, Low-Frequency Vibration Isolation Platform Adapts to Space Constraints in Critical Micro- and Nano-Microscopy
At about 2-1/2 inches in height, while isolating vibrations as low as 1 hertz, the new completely-passive Negative-Stiffness tabletop vibration isolation platform – developed by Minus K Technology – provides the industry’s thinnest low-height, low-frequency isolator for Microscopy (SPM, SEM, AFM, etc.), Micro-Hardness and Nano-Indenter Testing, Laser/Optical Systems, Biology/Neuroscience Systems, Spacecraft Ground Testing, Analytical Balances, Audio Reproduction, Vacuum & Cleanroom applications. - January 10, 2024 - Minus K Technology Inc.
Zifo Named Fastest Growing Technology Company in Deloitte Technology Fast 50 India 2023
Zifo has bagged this award for the 11th consecutive time. - December 20, 2023 - Zifo
ALPCO Announces the Commercial Launch of Its FDA 510(K) Cleared Calprotectin Immunoturbidimetric Assay
American Laboratory Products Company, Ltd. ("ALPCO"), a global provider of specialty diagnostic solutions, proudly announces the commercial launch of its FDA 510(k) cleared Calprotectin Immunoturbidimetric Assay, enhancing its suite of Gastrointestinal (GI) diagnostic solutions. In July... - December 19, 2023 - ALPCO
Huntington Study Group and Roche Collaborate on GENERATION HD2 Study
The Huntington Study Group together with its wholly-owned clinical research organization, HSG Clinical Research, Inc. (HSGCR), is pleased to announce a collaboration with Roche-Genentech to optimize our collective experiences in Huntington’s disease (HD) clinical research. Together we are... - December 13, 2023 - Huntington Study Group
Breakthrough Study by Microvascular Therapeutics Reveals Fibrin-Targeted Phase Shift Microbubbles as Promising Solution for Microvascular Obstruction
Microvascular Therapeutics, a trailblazing healthcare research and development company, announces a groundbreaking study on the treatment of Microvascular Obstruction (MVO) through sonoreperfusion. The study, led by Dr. John J. Pacella and his team at the Center for Ultrasound Molecular Imaging and... - December 13, 2023 - Microvascular Therapeutics, Inc.
Microvascular Therapeutics Selected as a Participant in the 2023 H7 BioCapital Accelerator Program
Microvascular Therapeutics (MVT), a clinical stage biopharma pioneering the potential of ultrasound in diagnostics and theranostics, has been selected to join the prestigious H7 Healthcare Accelerator 2023 Cohort, powered by H7 BioCapital. The program is renowned for providing a dynamic platform... - December 13, 2023 - Microvascular Therapeutics, Inc.
Creative Diagnostics Announces New Test Reagents for Dapsone
Creative Diagnostics has announced the launch of a range of new reagents for the analysis of Dapsone in food. - December 13, 2023 - Creative Diagnostics
Creative Diagnostics Announces New MBC Assay for Antibacterial Testing
Creative Diagnostics has announced the launch of its new Minimum Bactericidal Concentration (MBC) Assay for antibacterial testing. - December 13, 2023 - Creative Diagnostics
Creative Diagnostics Launches Macrophage Migration Inhibitory Factor Reagents for Research Applications
Creative Diagnostics has launched a portfolio of Macrophage Migration Inhibitory Factor (MIF) reagents for researchers. - December 13, 2023 - Creative Diagnostics
Announcing the Ales for ALS™ Roadtrip Across America
Over the coming months, Ales for ALS™ will be traveling across the country in an RV to connect with brewers and beer drinkers – all in support of ALS research. - December 04, 2023 - ALS Therapy Development Institute
FF Biotherapeutics Announces Search for Angel Investment to Revolutionize Immunotherapy and Vaccine Development
FF Biotherapeutics, a pioneering biotech startup at the forefront of immunotherapy and vaccine development, today announced its active pursuit of Angel investment. This critical funding round aims to propel the development of its groundbreaking Synthetic Standardized Convalescent Plasma (SSCP) and... - November 29, 2023 - FF Biotherapeutics